Quick-Glia™ Astrocytes – Human iPSC-Derived Astrocytes (Healthy Donor)
Our proprietary transcription factor-based stem cell differentiation method produces highly pure populations of astrocytes without a genetic footprint. Quick-Glia™ Astrocytes – Human iPSC-derived Astrocytes display typical astrocyte morphology and express markers such as S100 Calcium Binding Protein β (S100β), Chondroitin Sulfate Proteoglycan 8 (CD44), Aldehyde Dehydrogenase 1 Family Member L1 (ALDH1L1), and the mature astrocyte marker Glial Fibrillary Acidic Protein (GFAP). When handled and maintained according to the instructions in this user guide, astrocytes are viable long-term and are suitable for a variety of characterization and neurotoxicity assays.
$1,200.00
Advantages of iPSC-Derived Astrocytes
~ 1 Month Differentiation
Functionally Validated
Highly Pure Population
No Genetic Footprint
Patient-Derived Astrocytes
Quick-Glia™ Astrocytes are human, iPSC-derived astrocytes generated using our proprietary, transcription factor-based differentiation method. This non-integrating process results in highly pure, mature astrocytes with no genetic footprint offering a scalable, reproducible solution for CNS-focused drug discovery and neurobiology research.
These cells exhibit hallmark astrocytic morphology and express key markers such as GFAP, CD44, and ALDH1L1. Their functional capabilities, including glutamate uptake, enable relevant disease modeling and compound screening in vitro.
Astrocytes Protocol
Our user-friendly, optimized protocol supports robust and reproducible differentiation. This step-by-step guide outlines key culture stages and timelines for generating functional astrocytes from cryopreserved vials.


Astrocyte morphology is confirmed via phase contrast imagery: Representative phase contrast images of Quick-Glia™ Astrocytes – Human iPSC-derived Astrocytes on days 1, 2, 3, 6, 9, 14, 21, and 28 post-differentiation (scale bar = 100 μm).
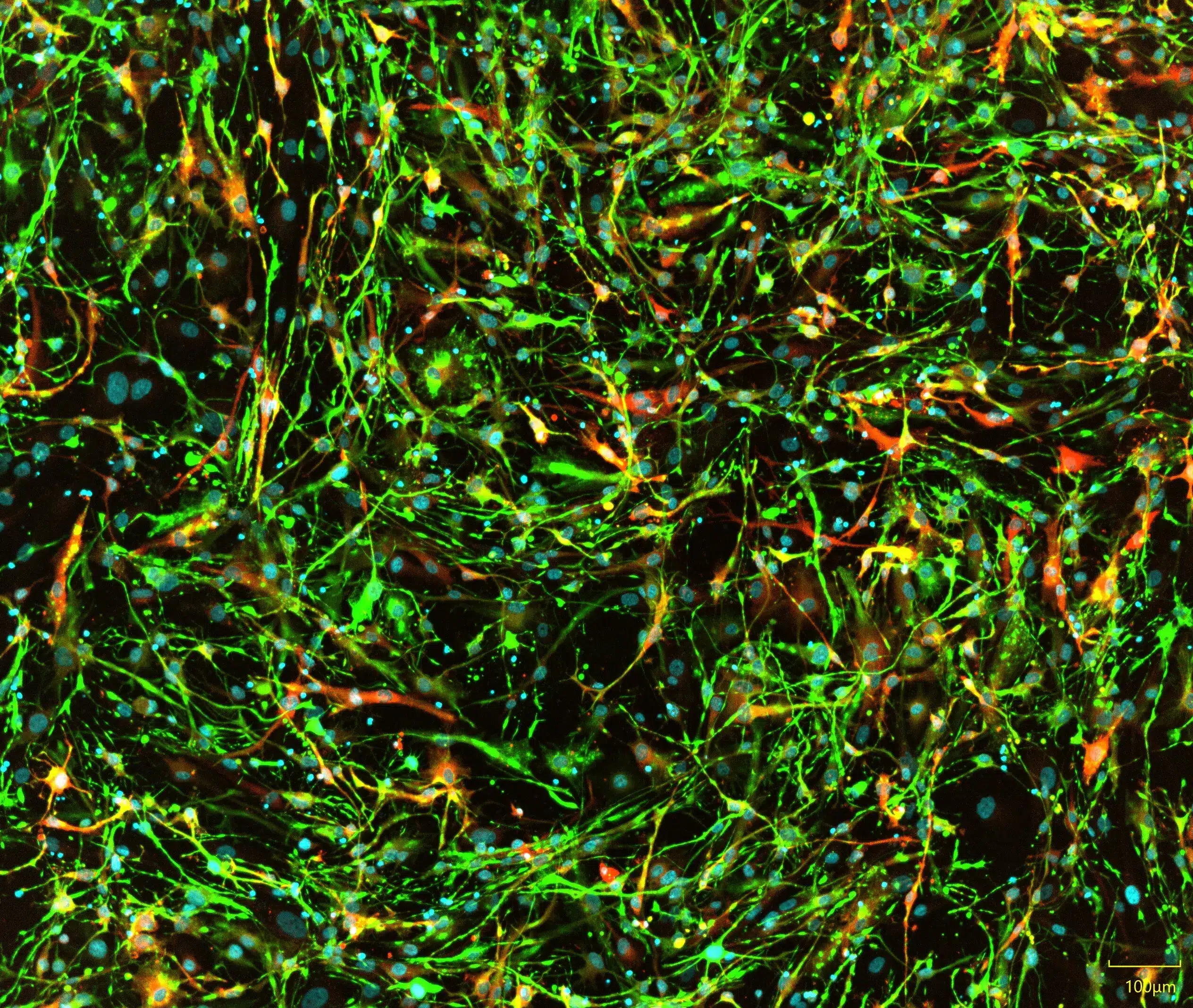
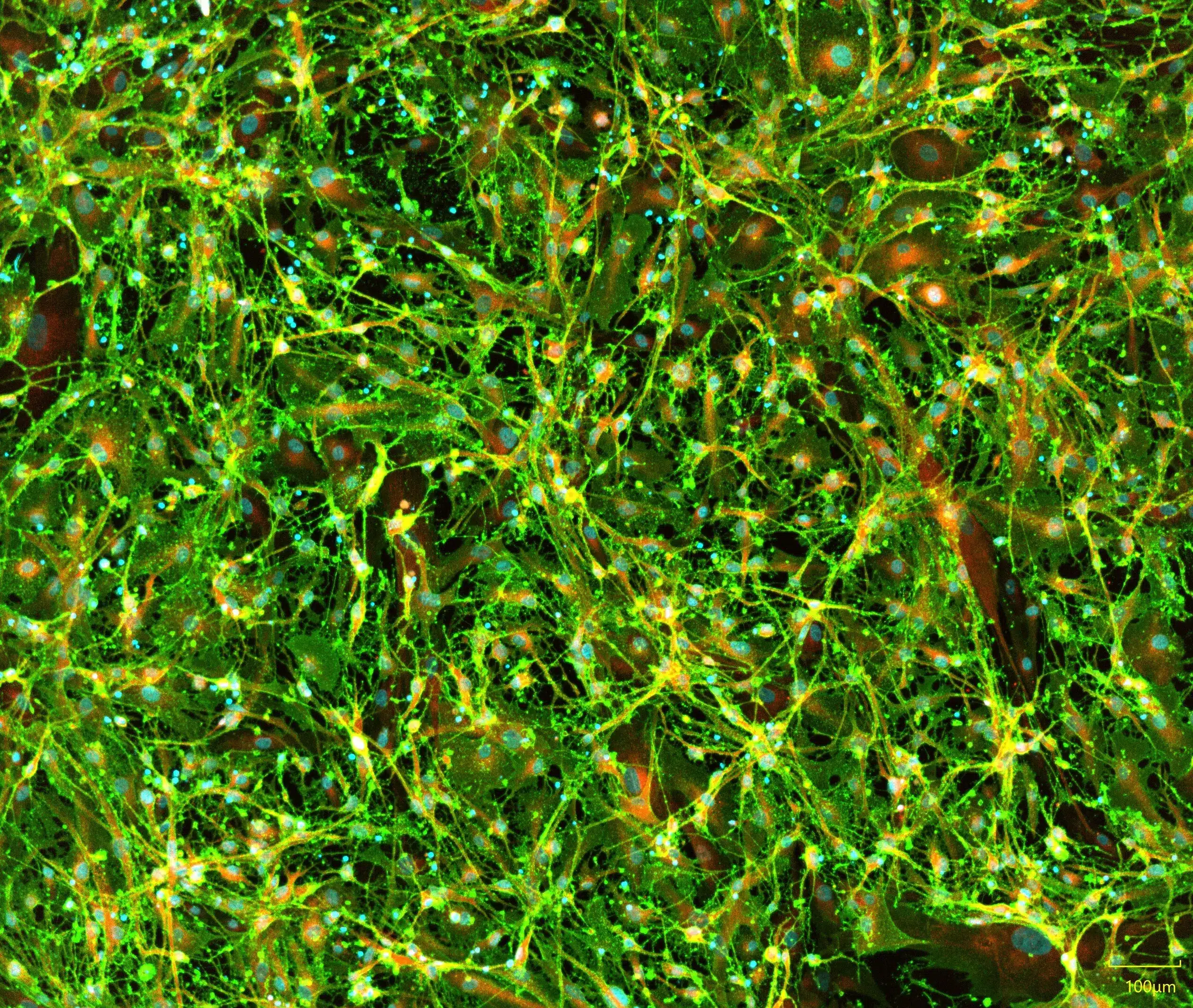
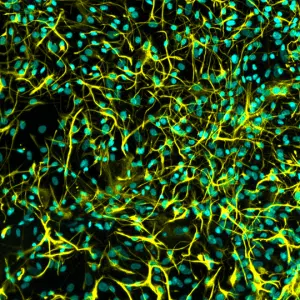
Astrocyte Marker Validation: GFAP, CD44, ALDH1L1
Immunocytochemistry confirms expression of key astrocytic markers in Quick-Glia™ cultures:
- GFAP – Mature astrocyte intermediate filament
- CD44 – Surface adhesion molecule
- ALDH1L1 – Astrocyte-specific cytoplasmic enzyme

iPSC-derived astrocytes express glial markers and display typical astrocytic morphology. Immunofluorescent staining of Quick-Glia™ Astrocyte shows expression of astrocytic markers CD44, ALDH1L1, and GFAP. Nuclei are counterstained with Hoechst 33342 (cyan) (scale bar = 100 μm).
Gene Expression Profile Compared to Primary Human Astrocytes

(A) Gene expression profiles of iPSCs and Quick-Glia™ – Astrocyte SeV Culture on day 28 were compared with the profile of human primary astrocytes and the results are shown as scatter plots. The horizontal axis indicates the expression levels of genes in human primary astrocytes purchased from ScienCell (Catalog Number: 1800-5), whereas the vertical axis indicates the expression levels of genes in iPSCs (left) and in Quick-Glia™ – Astrocyte SeV Culture on day 28 (right). The levels of gene expression are shown based on transcripts per million (TPM) in the log10 scale. Blue and green dots represent upregulated and downregulated genes (FDR<0.05), respectively, relative to their levels in human primary astrocytes. (B) Similarities of gene expression profiles of human iPSCs and Quick-Glia™ – Astrocyte SeV Culture on days 14, 28 and 42 to the profile of human primary astrocytes are shown as a bar chart. The vertical axis indicates Pearson correlation (r) based on median-subtracted logTPM.
Confirming Astrocyte Identity Via qPCR

Real-time quantitative PCR analysis of expression levels of astrocyte-associated genes CD44, GFAP, S100β and ALDH1L1 were examined. Graphs show comparison of Quick-Glia™ – Astrocyte SeV Culture on day 28 (A) and day 42 (B) with human brain total RNA (TaKaRa, Catalog Number: 636530). The relative gene expression is normalized to phosphoglycerate kinase 1 (PGK1), and then calculated as a fold induction relative to undifferentiated hPSCs as a control. Error bars show standard deviation.
Functional Validation: Glutamate Uptake Assay
Quick-Glia™ Astrocytes demonstrate active glutamate clearance, comparable to primary human astrocytes.

Human primary astrocytes (ScienCell Research Laboratories, Catalog number: 1800) and Quick-Glia – Astrocyte SeV kit cultures, harvested at either 28 days or 42 days post SeV infection, were plated at 7,800 cells/cm2 in a Geltrex-coated 96-well plate (Corning, Catalog number: 353072) and grown in ScienCell Astrocyte medium (ScienCell Research Laboratories, without FBS, Catalog number: 1801) for 14 days. As a negative control, human iPSCs were plated in StemFit Basic04 medium (Ajinomoto, Catalog number: ASB04-C) two days before assay measurement using the plating density stated above. 30 minutes prior to assay execution, culture medium was replaced with Hanks’ Balanced Salt Solution (HBSS) without phenol red (Fisher Scientific, Catalog number: 14025092). Subsequently, cells were exposed to 100µM L-glutamate (Tocris, Catalog number: 0218) in HBSS for 60 minutes. Immediately after the assay, solution was collected and cells were dislodged using a 1:1 mixture of TrypLE Select Enzyme (Fisher Scientific, Catalog number: 12563029) and 0.02% EDTA (Lonza, Catalog number: 17-711E). The numbers of live cells were determined using a NucleoCounter NC-200 (Chemometec, Catalog number: 900-0200) cell counter. Glutamate concentration was measured by a bioluminescence-based Glutamate-Glo assay kit (Promega, Catalog number: J7021) and a SPECTRAFluor Plus microplate reader (Tecan). To determine the amount of glutamate cleared, all values were first background signal-subtracted using a HBSS only sample and concentrations were thereafter acquired from the glutamate titration curve according to manufacturer’s instructions.
Product Specifications
| Parameters | Specifications |
|---|---|
| Product Name | Quick-Glia™ Astrocytes - Human iPSC-Derived Astrocytes |
| Catalog No. | AS-SeV-HC-CW50065 |
| Product Components | Cryopreserved cells and Component P |
| Starting Material | iPSCs derived from peripheral blood mononuclear cells (CIRM line CW50065) |
| Storage Conditions | Frozen cells should be stored in liquid nitrogen (vapor phase). The rest of the components should be stored at -20°C. |
| Cell Type | Astrocytes |
| Culture Type | Feeder Cell-Free |
| Disease | Healthy Donor |
| Donor Sex | Female |
| Donor Age At Sampling | 74 |
| Donor Race Ethnicity | Caucasian, not Latino |
| Patient History | See Resources section for more information. |
| Reprogramming Method | Episomal vector |
| Induction Method | Transcription factors delivered by Sendai virus |
| Growth Properties | Adherent |
| Number of viable cells | > 1.0 million viable cells per vial upon thawing |
| Cell viability and remaining live cells | >50% at day 1, >211 live cells per mm2 >50% at day 7, >211 live cells per mm2 |
| Differentiation | Log10 RQ Value: ALDH1L1 (>1), GFAP (>4), CD44 (>1.5), SLC1A3 (>1.5) |
| Sterility | No growth observed |
| Mycoplasma | No mycoplasmal enzymes detected |
| Morphological Observation | Cells are adherent and display characteristic astrocytic morphology, including stellate cell bodies and extended cytoplasmic processes, indicative of a differentiated phenotype. |
| Restricted Use | For research use only. Not for use in diagnostic or therapeutic procedures. |
Resources
Quick-Glia™ Astrocyte – SeV Kit
Antibody Validation with mRNA Transfection Into Human Pluripotent Stem Cells
Human iPSC-derived Excitatory Neurons and Astrocytes Differentiated by Quick-Tissue™ Technology for Functional Drug Screening on MEAs
Generation of Alzheimer’s Disease Model Using Human iPSC-derived Neurons and Astrocytes Produced by a Transcription Factor-Based Differentiation Protocol (Neuro4D 2022)
Using Human iPSC-derived Neurons and Astrocytes Produced by Transcription Factor-Based Differentiation Protocols to Generate Models to study Alzheimer’s Disease in vitro (Society for Neuroscience 2022)
Related Products
FAQs
Does Quick-Tissue™ technology leave a genetic footprint?
Sendai virus (SeV) is an RNA virus, so it does not integrate into the genomic DNA. In principle, a foreign gene introduced intracellularly in the form of RNA is quickly translated and expressed because, unlike DNA, RNA does not need to enter the nucleus for forced expression, thereby providing no chance of mutagenesis. This is discussed in the following review paper: Yamamoto, et al., (2009) “Current prospects for mRNA gene delivery.” Eur. J. Pharm Biopharm 71, 484-489.
Will SeV remain active after differentiation?
No. The SeV used in our kits is a temperature-sensitive mutant that is active at 33℃ but becomes inactive at 37℃, which is the temperature instructed in the user guides post-differentiation.
Is Sendai virus (SeV) dangerous?
SeV is not pathogenic to humans (i.e., humans are not the natural host of the virus) and the infection does not persist in immunocompetent animals. Furthermore, SeV used in our kits does not produce infectious viral particles upon transduction to host hPSCs and is a temperature-sensitive mutant, such that it is active at 33℃ but becomes inactive at 37℃. However, because SeV can be transmitted by aerosol and contact with respiratory secretions and is highly contagious, appropriate care must be taken to prevent potential mucosal exposure to the virus. Our SeV-based kits must be used under Biosafety Level 2 (BL-2) containment with a biological safety cabinet or a laminar flow hood and with appropriate personal protective equipment. In the event that the virus comes into contact with skin or eyes, decontaminate the affected area by flushing with plenty of water and follow the safety manual prepared by your laboratory and approved by your Institutional Biosafety Committee.
Do I need a license agreement for any of Ricoh Biosciences’ products?
No. You don’t need any licence or material transfer agreement (MTA) to use our differentiation kits or iPSC-derived cells. However, please be advised that these products are for research use only.
Contact Us
Have a question about our products, services, or custom projects? Our team is here to help—reach out and we’ll get back to you as soon as possible.
Subscribe
By signing up you are agreeing to our Privacy Policy