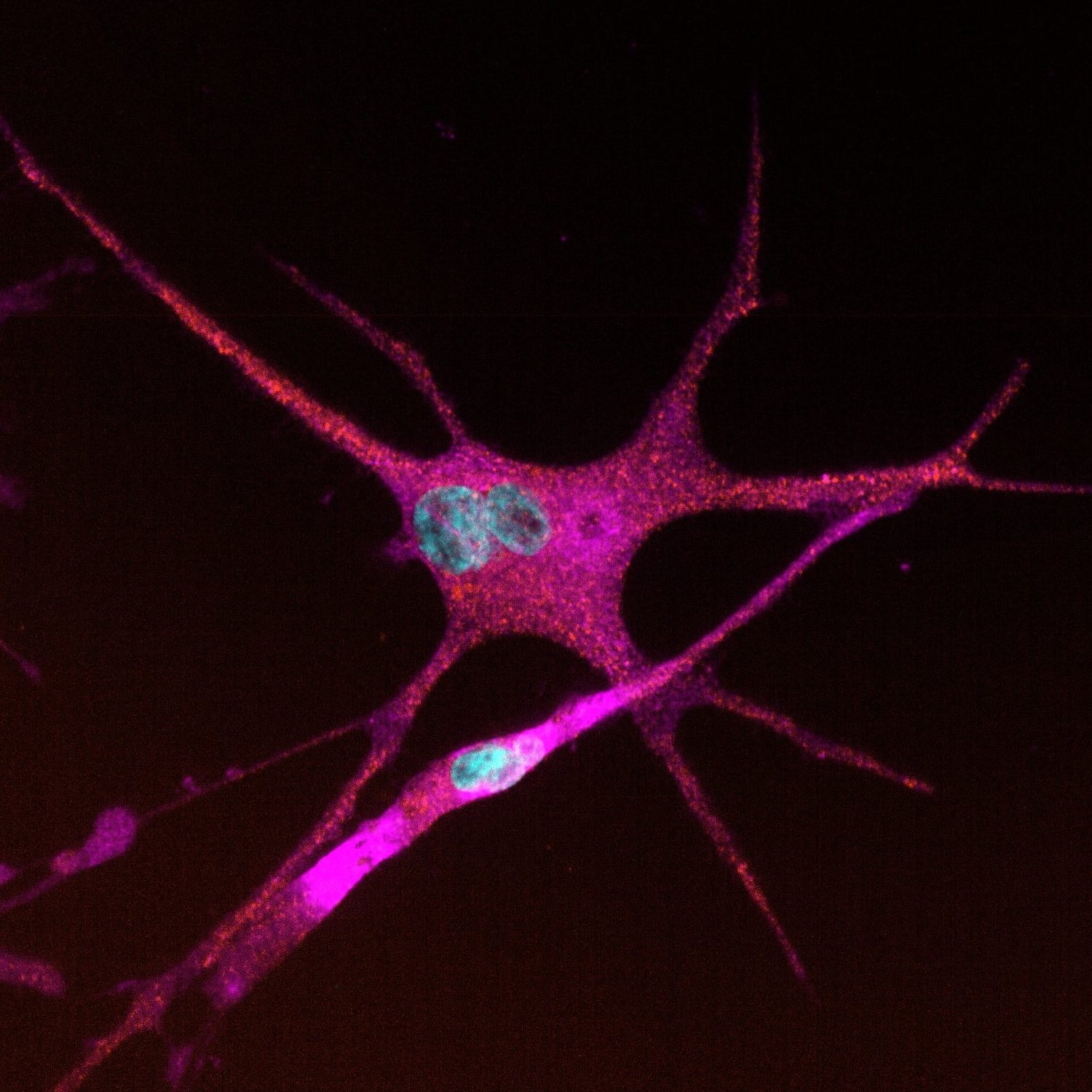
Quick-Glia™ Microglia – Human iPSC-Derived Microglia (Healthy Donor)
Quick-Glia™ Microglia – Human iPSC-Derived Microglia provide a consistent, biologically relevant model for studying neuronal function and disease. These cryopreserved, ready-to-use glial cells offer reproducibility for drug discovery, toxicity screening, and neurodegenerative disease research. Differentiated with our proprietary, footprint-free transcription factor-based technology, they mature rapidly and deliver robust performance.
Original price was: $999.00.$450.00Current price is: $450.00.
Advantages of iPSC-Derived Microglia
~ 1 Week Differentiation
Functionally Validated
Highly Pure Population
No Genetic Footprint
Patient-Derived Microglia
We offer microglia derived from human iPSCs licensed through The California Institute for Regenerative Medicine (CIRM). These glial cells have a diverse genetic background, as hiPSC lines from over 1,500 donors are available in the CIRM repository. If you require microglia from a specific donor, contact us to discuss your needs.
Microglia Protocol
Explore our detailed differentiation protocols, a step-by-step guide designed to simplify and optimize your laboratory procedures using our iPSC-derived cells and differentiation kits. These protocols leverage the latest advancements in iPSC technology to ensure efficient and reproducible results.


Microglia morphology is confirmed via phase contrast imagery: Representative images of Quick-Glia™ Microglia – Human iPSC-derived Microglia on days 1-7 post-thaw (scale bars = 100 μm).
Microglia Characterization
Microglia Marker Expression

iPSC-derived microglia express microglial markers and display typical morphology. Immunofluorescent staining of Quick-Glia™ – Human iPSC-derived Microglia culture shows cells with typical amoeboid morphology and expression of AIF1 (magenta) and TMEM119 (green) on day 7 post-thaw (scale bars = 100 μm).

RT-PCR analysis of marker expression in Quick-Glia™ Microglia – Human iPSC-Derived Microglia. Expression levels of CD45, CD68, CD44, AIF1, P2RY12, CX3CR1, and TREM2 were measured in iPSCs (baseline), differentiated microglia at day 8 post-thaw, and differentiated microglia without cryopreservation. Increased expression in both differentiated conditions confirms microglial identity.
Microglia Functionality
Phagocytosis is a core function of microglia and a key indicator of their physiological relevance in vitro. To assess the functional activity of our iPSC-derived microglia, we performed phagocytosis assays using pHrodo-labeled particles. These assays provide a visual and quantitative measure of microglial engulfment activity, with fluorescence intensity increasing in acidic compartments following particle internalization.
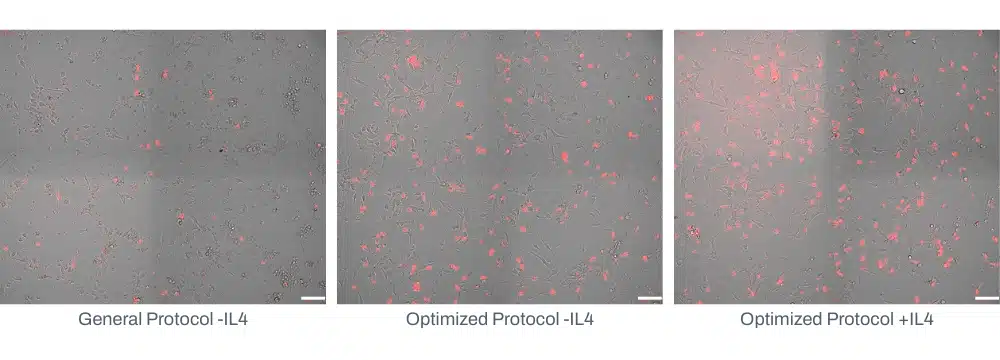
Functional validation of iPSC-derived microglia via pHrodo assay. Representative fluorescence microscopy images showing phagocytosis of pHrodo-labeled particles by Quick-Glia™ Human iPSC-Derived Microglia on Day 6 post-thaw. Increased intracellular fluoresnece indicates successful engulfment and acidification, confirming phagocytic activity (scale bars = 100 μm).
Product Specifications
| Parameters | Specifications |
|---|---|
| Product Name | Quick-Glia™ Microglia - Human iPSC-Derived Microglia |
| Catalog No. | MG-SeV-HC-CW50065 |
| Product Components | Cryopreserved cells, Component MG1, Component MG2, and Component MG3 |
| Starting Material | iPSCs derived from peripheral blood mononuclear cells (CIRM line CW50065) |
| Storage Conditions | Frozen cells should be stored in liquid nitrogen (vapor phase). The rest of the components should be stored at -20°C. |
| Cell Type | Microglia |
| Culture Type | Feeder Cell-Free |
| Disease | Healthy Donor |
| Donor Sex | Female |
| Donor Age At Sampling | 74 |
| Donor Race Ethnicity | Caucasian, not Latino |
| Patient History | See Resources section for more information. |
| Reprogramming Method | Episomal vector |
| Induction Method | Transcription factors delivered by Sendai virus |
| Growth Properties | Adherent |
| Number of viable cells | > 1.0 million viable cells per vial upon thawing |
| Cell viability and remaining live cells | >50% at day 1, >211 live cells per mm2 >50% at day 7, >211 live cells per mm2 |
| Differentiation | >75% IBA1 positive cells, >50% TMEM119 positive cells |
| Sterility | No growth observed |
| Mycoplasma | No mycoplasmal enzymes detected |
| Morphological Observation | Cells are adherent and display morphology consistent with microglial identity, including small cell bodies and cytoplasmic processes. |
| Restricted Use | For research use only. Not for use in diagnostic or therapeutic procedures. |
Resources
Patient-Derived iPSC Neuronal Models Reveal Alzheimer’s Disease-Associated Pathology and Provide a Platform for Drug Discovery (SfN 2025)
Quick-Glia™ Microglia – Maintenance Medium
Quick-Glia™ Microglia – Human iPSC-Derived Microglia
Predicting Clinical Efficacy with Alzheimer’s Disease iPSC Panels
Unlocking the Secrets of Alzheimer’s, Epilepsy, and More with Cutting-Edge iPSC Technology
Related Products
FAQs
What kind of transcription factors are used for differentiation induction?
It is a proprietary formulated RNA and cannot be disclosed.
Why do I see many floating cells and/or cellular debris on day 1?
hPSCs were likely damaged while they were harvested. hPSCs may have been mechanically harvested by scraping or were pipetted too much or too vigorously. Lift Solution D1-treated hPSCs only by pipetting them up and down up to 15 times. The mixing of hPSCs with SeV may have also been done too strongly. Gently rock the plate several times to mix hPSCs with SeV.
What should I do if I find on day 1 that cells were accumulated in the center of the well?
It is not recommended to start mRNA-based differentiation induction because the differentiation efficiency is expected to be quite low. For successful differentiation using our mRNA-based methods including Mesendoderm Booster, hPSCs should be evenly distributed in the well. It is likely that the plate was shaken too much, and cells were accumulated in the center of the well while they were floating. Next time, please handle the plate more gently or it can be left on a flat bench top without vibration for 15 min before it is put inside a CO₂ incubator.
What should I do if I find on day 1 that my culture was seeded only in the center but not in the periphery of the well?
Unless cells are accumulated in the center of the well, the instructions can be followed since this is an issue of coating. We have observed that 4-well plates available from Nunc sometimes do not allow cells to settle down in the periphery of the well when our coating conditions are applied. Next time, please use a 24-well plate and try the following: 1) incubate the plate with Coating Material A at 4°C overnight instead of 37°C for 2 hours, 2) do not aspirate the supernatant completely or leave the supernatant just enough to cover the well bottom surface after coating with Coating Material A is completed and 3) do not touch the bottom of wells with fingers when handling the plate.
I plated cells as instructed, so why does my culture look less confluent than the one shown in the user guide on day 1?
hPSCs were likely damaged while they were harvested. Ideally, hPSCs should be lifted only by pipetting several times using Solution D1 as instructed in the user guide. Even if pipetting 15 times does not lift all of the cells in the culture, do not attempt to do more pipetting but collect only cells that come off. Instead rinse the culture with PBS and start Solution D1 treatment again but with longer incubation (up to 20 minutes). Next time please reduce the concentration of the substrate used to coat the dish/well for the maintenance of the source hPSCs to 50-70%.
Why does my culture have colonies that appear balled up and round on day 1?
It could be that Coating Material A was diluted at room temperature or diluted using PBS warmed to room temperature. Keep both Coating Material A and PBS on ice before dilution.
Can I use an orbital shaker for SeV infection while incubating cell suspension for 10 minutes?
The purpose of flicking the tube containing cells and SeV every 2 minutes during the 10 minute incubation is to prevent cells from settling down and forming aggregates. If the orbital shaker can do so, then it is possible to use it.
Can I use Geltrex or Matrigel as a coating substrate for hPSC cultures?
Our kits are not optimized with these substrates. However, if your hPSC differentiation protocols are already optimized with such a substrate, you may try using one of these with our kit at your own risk.
I was able to harvest more hPSCs than I needed for the kit. Can I replate or freeze the leftover hPSCs to maintain their culture?
No. If you’re following the instructions given in the user guide, the hPSCs will be harvested in a differentiation medium so the leftover hPSCs cannot be maintained.
Why are there many aggregates after harvesting hPSCs?
After harvesting hPSCs, cells should not be left in the collection tube for more than 5 minutes. Leaving cells for a longer period may cause the formation of cellular clumps which interferes with infection. Single cells are best for infection.
Why doesn’t Solution D1 dissociate my hPSC culture without scraping?
The concentration of the substrate used to coat the dish/well was likely too high, so Solution D1 treatment requires longer incubation than 10 minutes (up to 20 minutes). Next time, please reduce the concentration of the substrate to 50-70%.
Why do differentiation kits require hPSCs to be maintained under StemFit or StemFlex conditions?
hPSCs should be adapted to passaging as single cells prior to differentiation. Our protocols have only been tested with StemFit/StemFlex, so we can’t guarantee results using other culture conditions.
How long do I need to maintain hPSCs prior to beginning to use your kit?
Before beginning differentiation, cultures should be healthy and displaying typical morphologies (i.e., round colonies with tightly packed cells exhibiting a small cytoplasmic area), few spontaneously differentiating cells, and normal growth rates (i.e., doubling once a day). We recommend growing the cells for at least 14 days post-thaw before differentiating and not allowing the cultures to reach more than 80% confluency.
Do I need a license agreement for any of Ricoh Biosciences’ products?
No. You don’t need any licence or material transfer agreement (MTA) to use our differentiation kits or iPSC-derived cells. However, please be advised that these products are for research use only.
Is Sendai virus (SeV) dangerous?
SeV is not pathogenic to humans (i.e., humans are not the natural host of the virus) and the infection does not persist in immunocompetent animals. Furthermore, SeV used in our kits does not produce infectious viral particles upon transduction to host hPSCs and is a temperature-sensitive mutant, such that it is active at 33℃ but becomes inactive at 37℃. However, because SeV can be transmitted by aerosol and contact with respiratory secretions and is highly contagious, appropriate care must be taken to prevent potential mucosal exposure to the virus. Our SeV-based kits must be used under Biosafety Level 2 (BL-2) containment with a biological safety cabinet or a laminar flow hood and with appropriate personal protective equipment. In the event that the virus comes into contact with skin or eyes, decontaminate the affected area by flushing with plenty of water and follow the safety manual prepared by your laboratory and approved by your Institutional Biosafety Committee.
Will SeV remain active after differentiation?
No. The SeV used in our kits is a temperature-sensitive mutant that is active at 33℃ but becomes inactive at 37℃, which is the temperature instructed in the user guides post-differentiation.
Does Quick-Tissue™ technology leave a genetic footprint?
Sendai virus (SeV) is an RNA virus, so it does not integrate into the genomic DNA. In principle, a foreign gene introduced intracellularly in the form of RNA is quickly translated and expressed because, unlike DNA, RNA does not need to enter the nucleus for forced expression, thereby providing no chance of mutagenesis. This is discussed in the following review paper: Yamamoto, et al., (2009) “Current prospects for mRNA gene delivery.” Eur. J. Pharm Biopharm 71, 484-489.
Contact Us
Have a question about our products, services, or custom projects? Our team is here to help—reach out and we’ll get back to you as soon as possible.
Subscribe
By signing up you are agreeing to our Privacy Policy