Quick-Neuron™ Excitatory – Human iPSC-Derived Neurons (Healthy Donor)
Our proprietary transcription factor-based stem cell differentiation method produces neurons without a genetic footprint. Quick-Neuron™ Excitatory – Human iPSC-derived Neurons display typical neurite outgrowth and express neuronal markers, such as TUBB3 (pan-neuronal) and vGLUT1 (glutamatergic). When thawed and maintained according to the instructions in the user guide, the iPSC-derived neurons are viable long-term and are suitable for a variety of characterization and neurotoxicity assays.
$850.00
Advantages of iPSC-Derived Excitatory Neurons
~ 1 Week Differentiation
Functionally Validated
Highly Pure Population
No Genetic Footprint
Excitatory Neuron Protocol
Explore our detailed differentiation protocols, a step-by-step guide designed to simplify and optimize your laboratory procedures using our iPSC-derived cells and differentiation kits. These protocols leverage the latest advancements in iPSC technology to ensure efficient and reproducible results.


Excitatory Neuron morphology is confirmed via phase contrast imagery. Representative phase contrast images of Quick-Neuron™ Excitatory – Human iPSC-derived Neurons on days 1-7 post-thaw (scale bars = 100 μm).
Excitatory Neuron Characterization
Excitatory Neuron Marker Expression

iPSC-derived excitatory neurons express neuronal markers and display typical neurite growth. Immunofluorescent staining of Quick-Neuron™ Excitatory – Human iPSC-derived Neurons on Day 7 post-thaw that shows expression of the pan-neuronal marker TUBB3 and the glutamatergic neuron marker vGLUT1 (scale bar = 100 μm).
Functional Electrophysiology of Human iPSC-Derived Excitatory Neurons
Whole-Cell Patch Clamp

Electrophysiological properties of iPSC-derived excitatory neurons. Whole-cell patch clamp of Quick-Neuron™ Excitatory – Human iPSC-derived Neurons 5-6 weeks post-thaw. (A) A brightfield image of the neurons measured. (B) Spontaneous action potentials were recorded. (C) Spontaneous excitatory postsynaptic current of neurons was detected by voltage clamp measurement at -70mV, indicating the formation of mature synapses. Data courtesy of E-PHY SCIENCE SAS.
Multi-Electrode Array (MEA)
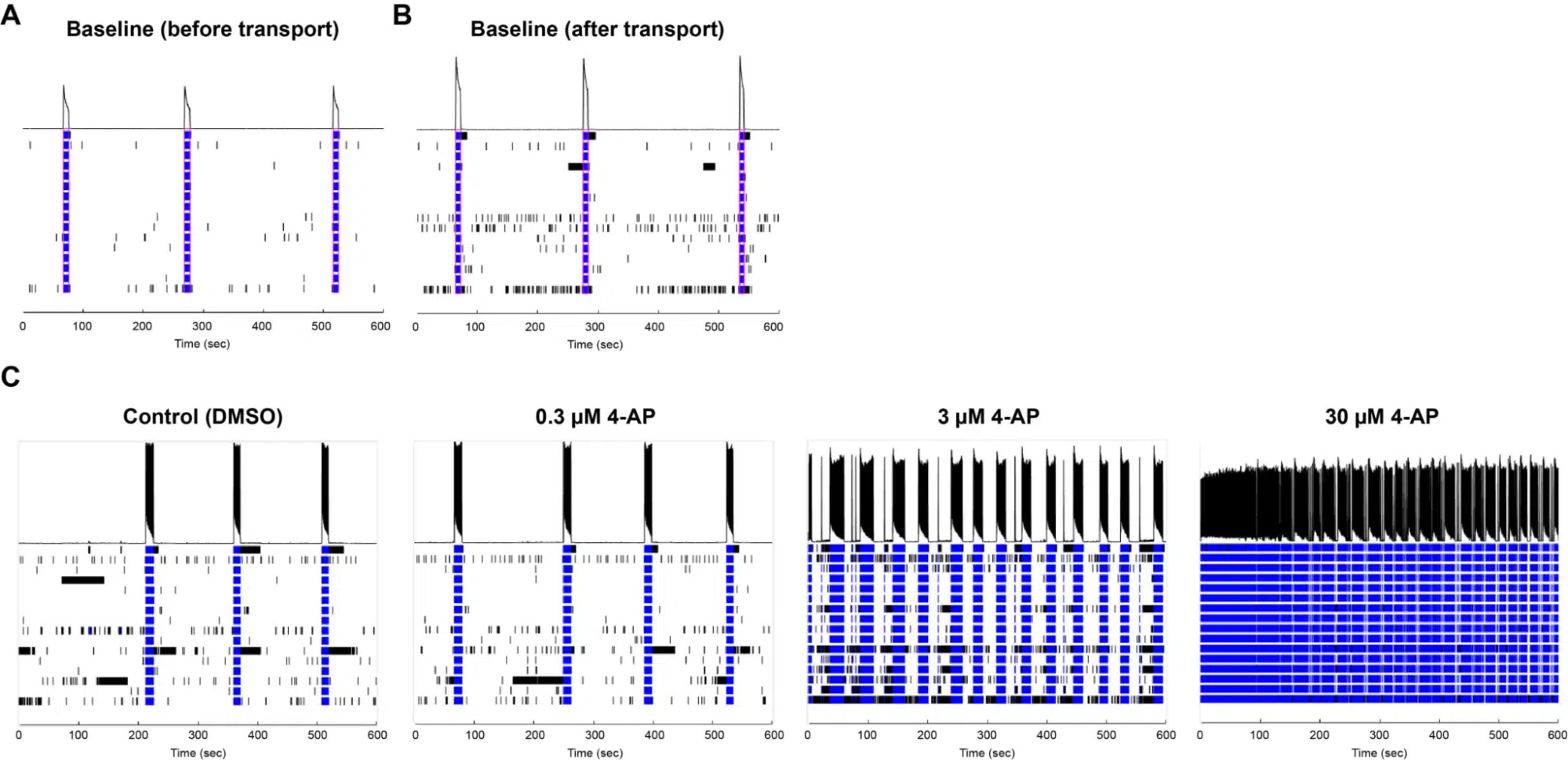
Excitatory Neurons seeded onto MEA plates remain functional after transport. An MEA plate seeded with Quick-Neuron™ Excitatory Neurons and primary human astrocytes was transported from Tokyo, Japan to Maryland, USA. The baseline network burst firing of one well was measured on day 49 post-thaw before transport (A) and on day 50 after 33-hr transport (B). (C) Network burst firing on day 51 exhibited responses to varying concentrations of 4-AP after transport. Data courtesy of Ricoh.
Transcriptomic Validation of Human iPSC-Derived Excitatory Neurons
RNA-Sequencing

Gene Expression of Quick-Neuron™ Excitatory Neurons. A heat map of selected gene expression data from RNA-seq performed on undifferentiated iPSCs, Quick-Neuron™ Excitatory Neurons (EX only) cultured for 10 days and 38 days, Quick-Neuron™ Excitatory Neurons cocultured with primary astrocytes (EX+AST) for 18 and 52 days, and primary fetal and adult brain samples is shown. Values represent log10(TPM + 1). Data courtesy of Ricoh.
Principal Component Analysis (PCA)

Quick-Neuron™ Excitatory Neurons display gene expression profiles similar to those of human brain: RNA-seq was performed on undifferentiated iPSCs, Quick-Neuron™Excitatory Neurons (EX only) cultured for 10 days and 38 days, Excitatory neurons cocultured with primary astrocytes (EX+AST) for 18 and 52 days, and primary fetal and adult brain samples. Principal component analysis indicates that Quick-Neuron™ Excitatory Neurons display gene expression similar to that of the human brain, particularly when grown with astrocytes. As cells remain in culture over time they more closely resemble adult human brain cells. Data courtesy of Ricoh.
Product Specifications
| Parameters | Specifications |
|---|---|
| Product Name | Quick-Neuron™ Excitatory - Human iPSC-derived Neurons |
| Catalog No. | EX-SeV-HC-CW50065 |
| Product Components | Cryopreserved cells, Component N, Component G2, and Component P |
| Starting Material | iPSCs derived from peripheral blood mononuclear cells (CIRM line CW50065) |
| Storage Conditions | Frozen cells should be stored in liquid nitrogen (vapor phase). The rest of the components should be stored at -20°C. |
| Cell Type | Excitatory Neurons |
| Culture Type | Feeder Cell-Free |
| Disease | Healthy Donor |
| Donor Sex | Female |
| Donor Age At Sampling | 74 |
| Donor Race Ethnicity | Caucasian, not Latino |
| Patient History | See Resources section for more information. |
| Reprogramming Method | Episomal vector |
| Induction Method | Transcription factors delivered by Sendai virus |
| Growth Properties | Adherent |
| Number of viable cells | > 1.0 million viable cells per vial upon thawing |
| Cell viability and remaining live cells | >50% at day 1, >211 live cells per mm2 >50% at day 7, >211 live cells per mm2 |
| Differentiation | At day 7 post-differentiation (CW50065) >80% TUBB3 positive cells >50% VGLUT1 positive cells among TUBB3 positive cells |
| Sterility | No growth observed |
| Mycoplasma | No mycoplasmal enzymes detected |
| Morphological Observation | Cells are adherent and neurites exhibit substantial outgrowth, elongation and branching, indicative of a differentiated phenotype. |
| Restricted Use | For research use only. Not for use in diagnostic or therapeutic procedures. |
Resources
Episcopic Brightfield Imaging of Neuronal Cells on High-Density Microelectrode Arrays Enables Prediction of Cell Region Through AI Learning
Quick-Neuron™ Excitatory – Maintenance Medium
Quick-Neuron™ Excitatory – Human iPSC-Derived Neurons
Genetic and Functional Profiling of hiPSC-derived Excitatory Neurons Differentiated by Quick-Neuron™ Technology
Visualizing Neurite Outgrowth by Lentiviral Transduction of Fluorescent Proteins into Human iPSC-Derived Excitatory Neurons
Related Products
FAQs
What should I do if I find on day 1 that cells were accumulated in the center of the well?
It is not recommended to start mRNA-based differentiation induction because the differentiation efficiency is expected to be quite low. For successful differentiation using our mRNA-based methods including Mesendoderm Booster, hPSCs should be evenly distributed in the well. It is likely that the plate was shaken too much, and cells were accumulated in the center of the well while they were floating. Next time, please handle the plate more gently or it can be left on a flat bench top without vibration for 15 min before it is put inside a CO₂ incubator.
Does Quick-Tissue™ technology leave a genetic footprint?
Sendai virus (SeV) is an RNA virus, so it does not integrate into the genomic DNA. In principle, a foreign gene introduced intracellularly in the form of RNA is quickly translated and expressed because, unlike DNA, RNA does not need to enter the nucleus for forced expression, thereby providing no chance of mutagenesis. This is discussed in the following review paper: Yamamoto, et al., (2009) “Current prospects for mRNA gene delivery.” Eur. J. Pharm Biopharm 71, 484-489.
Why do I see many floating cells and/or cellular debris on day 1?
hPSCs were likely damaged while they were harvested. hPSCs may have been mechanically harvested by scraping or were pipetted too much or too vigorously. Lift Solution D1-treated hPSCs only by pipetting them up and down up to 15 times. The mixing of hPSCs with SeV may have also been done too strongly. Gently rock the plate several times to mix hPSCs with SeV.
Will SeV remain active after differentiation?
No. The SeV used in our kits is a temperature-sensitive mutant that is active at 33℃ but becomes inactive at 37℃, which is the temperature instructed in the user guides post-differentiation.
Is Sendai virus (SeV) dangerous?
SeV is not pathogenic to humans (i.e., humans are not the natural host of the virus) and the infection does not persist in immunocompetent animals. Furthermore, SeV used in our kits does not produce infectious viral particles upon transduction to host hPSCs and is a temperature-sensitive mutant, such that it is active at 33℃ but becomes inactive at 37℃. However, because SeV can be transmitted by aerosol and contact with respiratory secretions and is highly contagious, appropriate care must be taken to prevent potential mucosal exposure to the virus. Our SeV-based kits must be used under Biosafety Level 2 (BL-2) containment with a biological safety cabinet or a laminar flow hood and with appropriate personal protective equipment. In the event that the virus comes into contact with skin or eyes, decontaminate the affected area by flushing with plenty of water and follow the safety manual prepared by your laboratory and approved by your Institutional Biosafety Committee.
Do I need a license agreement for any of Ricoh Biosciences’ products?
No. You don’t need any licence or material transfer agreement (MTA) to use our differentiation kits or iPSC-derived cells. However, please be advised that these products are for research use only.
Contact Us
Have a question about our products, services, or custom projects? Our team is here to help—reach out and we’ll get back to you as soon as possible.
Subscribe
By signing up you are agreeing to our Privacy Policy



